|
Case Report
CD56, CD68 positive double hit, diffuse large B-cell/high-grade B-cell lymphoma with rearrangement of MYC and BCL2 genes
1 Hemathopathology, Clinical Pathology Laboratory, INCYTE Diagnostics, Spokane, WA, USA
2 Surgical Pathology/Dermatopathology, Anatomic Pathology Laboratory, INCYTE Diagnostics, Tukwila, WA, USA
3 Laboratory Assistant, Anatomic Pathology Laboratory, INCYTE Diagnostics, Tukwila, WA, USA
Address correspondence to:
Solomon Tessega
Sea Tac, WA,
USA
Message to Corresponding Author
Article ID: 100013P03JA2025
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Andrews J, Long T, Tessega S. CD56, CD68 positive double hit, diffuse large B-cell/high-grade B-cell lymphoma with rearrangement of MYC and BCL2 genes. Edorium J Pathol 2025;9(1):1–5.ABSTRACT
Introduction: This is a report of diffuse large B-cell/high-grade B-cell lymphoma (DLBCL/HGBL)-MYC/BCL2 with CD56 and CD68 immunoreactivity, pseudopapillary architecture, and pleomorphic nuclei with multinucleation arising in the nasal cavity. This combination of features makes this a highly unusual case that contributes to our understanding of rare histopathologic and immunophenotypic features in DLBCL.
Case Report: A 64-year-old male has been diagnosed with double-hit, diffuse large B-cell lymphoma (DLBCL). Pathological examination revealed large, variably multinucleated anaplastic cells with multilobulated nuclei surrounding a vascular core and exhibiting a pseudopapillary growth pattern, all set against reactive lymphocytes and histiocytes. Further studies showed the tumor’s positivity for CD56 and CD68, along with other positive immunohistochemical (IHC) markers.
Conclusion: Additional investigations could contribute to understanding the clinical, prognostic, and diagnostic significance of unusual pathologic features in DLBCL and its subtypes.
Keywords: CD56, CD68, Diffuse large B-cell lymphoma, Immunohistochemistry, Pseudopapillary growth pattern
INTRODUCTION
This is a report of DLBCL/HGBL-MYC/BCL2 with CD56 and CD68 immunoreactivity, pseudopapillary architecture, and pleomorphic nuclei with multi-lobulation arising in the nasal cavity. This combination of features makes this a highly unusual case that contributes to our understanding of rare histopathologic and immunophenotypic features in DLBCL.
CASE REPORT
A 64-year-old male presented to the emergency department with one month of intractable epistaxis. He also reported headaches, right-sided eye pain, fullness in both ears, sinus pressure, pain in the right maxillary sinus, and bilateral earaches. He denied weight loss, night sweats, fever, chills, and fatigue. His physical examination revealed a body temperature of 98.3°F and tenderness over the right maxillary, ethmoid, and frontal sinuses. He received supportive care and was scheduled for further imaging in two weeks.
At his follow-up appointment, his symptoms persisted. Endoscopy revealed a mass in the right nasal cavity and middle meatus, extending into the nasal floor and the posterior choana. A maxillofacial CT scan showed a large enhancing polypoid mass, approximately 3.4 × 4.2 × 3.8 cm, in the right middle and inferior meatus, extending to the right maxillary ostium. No definitive lymphadenopathy was detected.
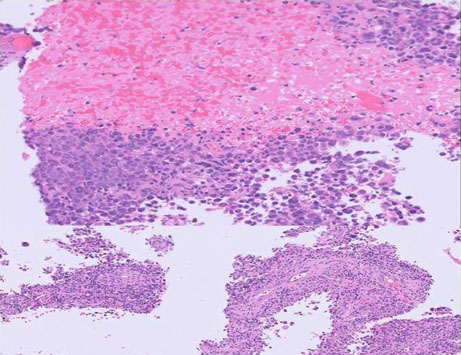
An endoscopic excisional biopsy was performed. Pathological examination revealed large, variably multinucleated anaplastic cells with multilobulated nuclei surrounding a vascular core exhibiting a pseudopapillary growth pattern with a background of reactive lymphocytes and histiocytes (Figure 1).
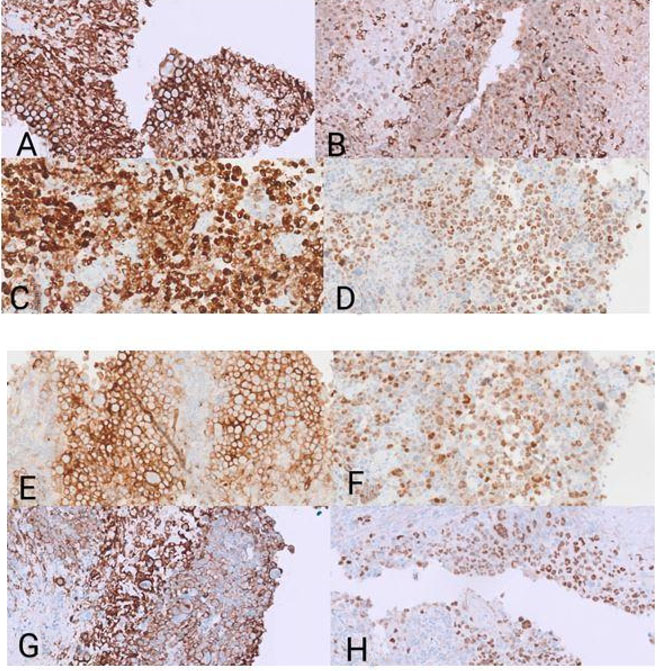
Immunohistochemistry showed bright positivity for CD56, CD45, CD20, BCL2, CD10, and dim expression of CD68 (Figure 2). There were variable expressions of BCL-6, MUM1, and PAX5. The Ki-67 proliferation marker labeled approximately 50–60% of the malignant cells. The cells were negative for T-cell markers (CD2, CD3, CD5, CD7, CD84, TIA-1, and CD43), CD30, ALK protein, EBER ISH, and CD138. Kappa and Lambda immunohistochemical stains were non-contributory. Flow cytometry failed to detect the malignant B-cells. This is due to their large size and cohesive growth pattern. The two primary differential diagnoses for this case are Extranodal NK/T-cell lymphoma, nasal type, which would stain positive for CD3 and EBER and negative for CD20, CD79a, and PAX5, and carcinoma/neuroendocrine carcinoma with a papillary growth pattern, which would stain positive for Pancytokeratin (AE1/AE3) and negative for lymphoid stains.
Fluorescence in situ hybridization (FISH) analysis, using a high-grade/large B-cell lymphoma-specific set of probes, revealed BCL2 (1-3R1-2G1F, 34%) and MYC (8q24.21)(1R1-2G−2F, 30%) gene rearrangements. Cerebrospinal fluid (CSF) was negative for malignant cells. The patient had extensive and aggressive disease. Therefore, we had planned on six cycles of dose-adjusted Rituximab, Etoposoid, Perdinison, Oncovin, Cyclophosmide, Hydroxydaunorubucine (DA R-EPOCH) with the potential for subsequent high-dose methotrexate for central nervous system (CNS) prophylaxis. After four cycles of dose-adjusted R-EPOCH an interval, positron emission tomography (PET) demonstrated a complete response with no residual disease appreciated (some bone marrow avidity was appreciated, but this was most consistent with hematopoiesis). He completed cycle five but subsequently developed grade 3 mucositis, and ultimately, he declined to complete his last cycle of DA R-EPOCH or any further therapy. He is currently three months out from his previous cycle with no clinical evidence of disease recurrence.
DISCUSSION
Diffuse large B-cell lymphoma (DLBCL) is the most common adult non-Hodgkin lymphoma (NHL) and is known for its high invasiveness and poor prognosis. It has two main biologically distinct molecular subtypes: germinal center B-cell-like (GCB) and activated B-cell-like (ABC) based on antigen expression patterns [1]. A subset of diffuse large B-cell lymphomas has cMYC and BCL2 gene rearrangements and was previously called double-hit (DH) lymphoma.
The latest revision of the WHO’s classification of hematolymphoid tumors renamed high-grade B-cell lymphoma with dual rearrangements of MYC and BCL2 and/or BCL6 as diffuse large B-cell lymphoma/high-grade B-cell lymphoma with MYC and BCL2 rearrangements (DLBCL/HGBL-MYC/BCL2) [2]. The HGBL-DH/TH entity comprises 13.3% of GCB and 1.7% of ABC, DLBCL cases. Additionally, HGBL-DH holds a poor prognosis and treatment outcome [3],[4].
CD56, also known as the neural cell adhesion molecule (NCAM), is a member of the immunoglobulin superfamily. Its expression in B-cell NHL is unusual. Moreover, there are only a few reported cases of CD56-positive HGBL-DH. One of the most extensive case series to date reported that CD56-positive DLBCL comprises only 3% of cases, totaling seven instances. The median age at diagnosis was 54.5 years, ranging from 30 to 57. The majority (five cases) were diagnosed as DLBCL, Germinal center (GC) type, while two were classified as DLBCL non-GC (ABC) type. One DLBCL of non-GC (ABC) type showed a double-expression profile with BCL2 and MYC protein expression. This study found that 28 out of 36 (76%) were of GC type, and the remaining 24% were non-GC (ABC) type. One reported case was double-hit lymphoma with translocations of MYC and BCL6 [5]. Our case is GC type, similar to most cases reported, but it also expresses BCL2 and MYC.
Another comprehensive case review encompassing ten reports and 35 CD56-positive DLBCL cases revealed a higher prevalence among males (3:1 ratio) and a wide age range from 15 to 87 years (mean age: 56.5 years). Among the 31 cases with available clinical data, only one occurred in the nasal cavity [6]. More literature reviews showed the association between CD56 positivity and extranodal involvement [7],[8]. Kawasaki et al. reported a possible propensity of CD56-positive DLBCL to infiltrate CNS. Our patient’s CSF flow analysis yielded no abnormalities [9].
Another unique characteristic of our case was the multilobulated nuclear features, dim expression of CD68, and pseudopapillary growth patterns, which are all unusual in DLBCL. In a study of 298 CD68-positive, NHL cases, 8 were large B-cell lymphomas (5-centroblastic and 3-immunoblastic) [10]. The prognostic significance of CD68 expression remains controversial. Most studies suggest that high CD68 expression is associated with a poor prognostic outcome compared to low expression [11],[12]. Cases expressing CD56 and CD68 are notably absent in the reviewed literature.
CONCLUSION
Certain features, such as CD56 and CD68 expression, may have a unique clinical presentation and prognostic outcome in DLBCL and its subtypes, such as DLBCL/HGBL-MYC/BCL2. Further investigation could contribute to understanding the clinical, prognostic, and diagnostic significance of these unusual pathologic features in DLBCL and its subtypes.
REFERENCES
1.
Hu S, Xu-Monette ZY, Balasubramanyam A, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: A report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2013;121(14):2715–24. [CrossRef]
[Pubmed]

2.
Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022;36(7):1720–48. [CrossRef]
[Pubmed]

3.
Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood 2018;131(18):2060–4. [CrossRef]
[Pubmed]

4.
Ennishi D, Jiang A, Boyle M, et al. Double-hit gene expression signature defines a distinct subgroup of germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 2019;37(3):190–201. [CrossRef]
[Pubmed]

5.
Gasljevic G, Boltezar L, Novakovic S, Setrajcic-Dragos V, Jezersek-Novakovic B, Kloboves-Prevodnik V. CD56-positive diffuse large B-cell lymphoma: Comprehensive analysis of clinical, pathological, and molecular characteristics with literature review. Radiol Oncol 2023;57(2):249–56. [CrossRef]
[Pubmed]

6.
Gu MJ, Ha JO. CD56 positive diffuse large B-cell lymphoma: A case report and literature review. Int J Clin Exp Pathol 2013;6(12):3023–5.
[Pubmed]

7.
Wang J, Zhang W, Ding W, et al. Diffuse large B-cell lymphoma with aberrant expression of CD56: A clinicopathologic and immunohistochemical study. [Article in Chinese]. Zhonghua Bing Li Xue Za Zhi 2016;45(2):78–82. [CrossRef]
[Pubmed]

8.
Gomyo H, Kajimoto K, Miyata Y, et al. CD56-positive diffuse large B-cell lymphoma: Possible association with extranodal involvement and bcl-6 expression. Hematology 2010;15(3):157–61. [CrossRef]
[Pubmed]

9.
Kawasaki T, Suzuki M, Sato A, et al. Neural cell adhesion molecule (CD56)-positive B cell lymphoma of the urinary bladder. J Clin Pathol 2016;69(1):89–92. [CrossRef]
[Pubmed]

10.
Carbone A, Gloghini A, Volpe R, Pinto A. KP1 (CD68)-positive large cell lymphomas: A histopathologic and immunophenotypic characterization of 12 cases. Hum Pathol 1993;24(8):886–96. [CrossRef]
[Pubmed]

11.
Li YL, Shi ZH, Wang X, Gu KS, Zhai ZM. Tumor-associated macrophages predict prognosis in diffuse large B-cell lymphoma and correlation with peripheral absolute monocyte count. BMC Cancer 2019;19(1):1049. [CrossRef]
[Pubmed]

12.
Cai QC, Liao H, Lin SX, et al. High expression of tumor-infiltrating macrophages correlates with poor prognosis in patients with diffuse large B-cell lymphoma. Med Oncol 2012;29(4):2317–22. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Jared Andrews - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Thomas Long - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Solomon Tessega - Conception of the work, Design of the work, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2025 Jared Andrews et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.