|
Case Report
Primary diffuse large B-cell lymphoma of the orbit: Case report
1 Resident, Department of Anatomopathology, Ibn Rochd University Hospital Center, Hassan II University of Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco
2 Resident, Department of Stomatology Oral and Maxillofacial Surgery, 20 August 1953, Hassan II University of Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco
3 Professor, Department of Anatomopathology, Ibn Rochd University Hospital Center, Hassan II University of Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco
4 Professor, Department of Stomatology Oral and Maxillofacial Surgery, 20 August 1953, Hassan II University of Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco
5 Professor and Chief of Anatomopathology Department, Ibn Rochd University Hospital Center, Hassan II University of Casablanca, Faculty of Medicine and Pharmacy, Casablanca, Morocco
Address correspondence to:
Siham Nagib
Casablanca, Morocco,
Message to Corresponding Author
Article ID: 100012P03SN2024
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Nagib S, Abatourab M, Nihad DSF, Opoko U, Benayad S, Slimani F, Karkouri M. Primary diffuse large B-cell lymphoma of the orbit: Case report. Edorium J Pathol 2024;8(1):1–7ABSTRACT
Introduction: Ocular adnexal lymphoma is a rare disease, the diagnosis may masquerade as orbital inflammatory disease, which can be challenging for both of pathologist and ophthalmologist. Therefore, it can progress rapidly to complete visual loss if the diagnosis was made tardily.
Case report: We report a case of primary diffuse large B-cell lymphoma (DLBCL) of the orbit in a 66-year-old man without history of lymphoma, who presented progressive decrease of visual acuity of his left eye, for three months. The clinical examination showed ptosis of the left eye, exophthalmos, swelling, and diplopia without sensory-motor disorder. Magnetic resonance imaging showed an intraconal soft tissue mass, poorly limited infiltrating superior and inferior rectus muscles and around the optic nerve without invading it, nor bone structures. Inflammatory pseudotumor was suggested. Histopathologic evaluation of incisional biopsy revealed infiltration of orbital soft tissue by sheets of dyscohesive, round and large lymphomatoid cells in a diffuse pattern. The tumoral lymphomatoid cells showed expression of CD20, Bcl2, Bcl6, CD10, and MUM1. The Ki-67 proliferation index was estimated at 80%, and they were negative for CD3 and CD5. On the basis of histological results and the absence of other tumor localization, the diagnosis of primary diffuse large B-cell lymphoma of orbit was retained. The patient was referred to the hematologist-oncologist. He underwent a positron emission tomography-computed tomography (PET/CT) scan, which showed no other localization, and he received chemotherapy with a complete remission and a good tolerance to the treatment.
Conclusion: Diffuse large B-cell lymphoma (DLBCL) is the second-most common non-Hodgkin’s lymphoma (NHL) in orbit, affected usually elderly patients (average age 69), with no sex predilection. It can manifest as exophthalmos, pain, decreased visual acuity, diplopia, and ptosis, and it has often a poor prognosis.
Keywords: Diffuse large B-cell lymphoma, Histology, Ocular adnexal lymphoma, Primary orbital lymphoma
INTRODUCTION
Ocular adnexal lymphoma (OAL) is a rare disease. It can occur primarily, defined as a lymphoma without evidence of concomitant systemic lymphoma and without history of lymphoma [1]; or secondary resulting from systemic disease.
It accounts for 1–2% of all non-Hodgkin’s lymphomas (NHLs) and 5–10% of all extranodal NHLs, but it represents the most prevalent malignant orbital tumor in older adults [2].
Although still rare, the incidence of orbital lymphoma has increased in the recent years [3],[4].
In order of frequency of ocular adnexal lymphoma sites, the orbit represents 37% of cases, followed by conjunctiva (29%); lacrimal system (20%) and eyelid (14%). The eyelid has the highest proportion of secondary lymphomas, accounting for 49% of all lymphoproliferative lesions of the eyelid [1].
Orbital lymphoma is often of B-cell origin (97%) but a few cases of T-cell lymphoma have been reported (3%). The most common B-cell lymphoma is extranodal marginal zone B-cell lymphoma (EMZL) (59%), followed by diffuse large B-cell lymphoma (DLBCL) (23%) [3].
The diagnosis of orbital lymphoma may masquerade as orbital inflammatory disease, which can be challenging for both of pathologist and ophthalmologist [5]. Therefore, it can progress rapidly to complete visual loss if the diagnosis was made tardily [6].
In this paper, we report a case of primary diffuse large B-cell lymphoma of the orbit. Given its rarity and primary nature, the initial diagnosis, based on clinical and radiological features, was that of an inflammatory pseudotumor. Here we will discuss its clinicoradiological, pathological, and immunohistochemical features.
CASE REPORT
A 66-year-old man, with no specific history, who presented progressive decrease of visual acuity of his left eye, for three months. He was in good general condition with no other associated symptoms and without history of lymphoma.
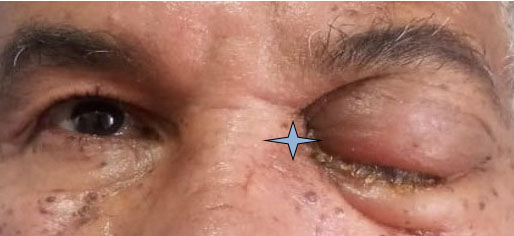
The clinical examination showed left periorbital tumefaction (upper and lower palpebral), with closure of the palpebral fissure, and exophthalmos, associated with chemosis and ptosis of the left upper eyelid (Figure 1). Ophthalmological examination showed limited ocular motility, visual acuity was reduced to a simple negative light perception (NLP), ocular tone was normal, and the fundus could not be examined due to an opalescent lens. The contralateral globe was normal. There was no regional lymph node involvement or neither bone erosion.
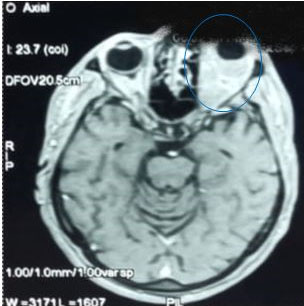
Brain magnetic resonance imaging (MRI) with brain and orbits protocol was performed after gadolinium injection, showed an intraconal soft tissue mass, oval and poorly limited, hypointense on T1W, and slightly hyperintense on T2W. That mass infiltrates superior and inferior rectus muscles and around the optic nerve without invading it, nor bone structures (Figure 2). Inflammatory pseudotumor was suggested.
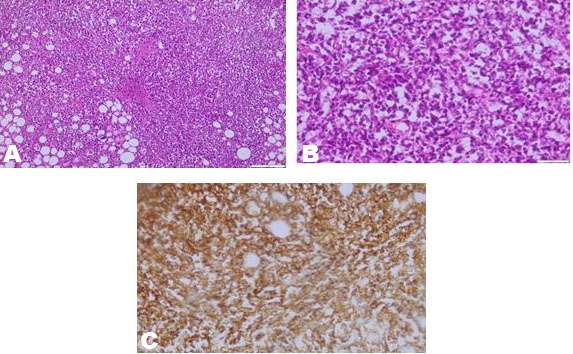
Histopathologic evaluation of incisional biopsy revealed infiltration of orbital soft tissue by sheets of dyscohesive, round and large lymphomatoid cells in a diffuse pattern (Figure 3). The cells had scant to mild amount of eosinophilic cytoplasm, large nuclei with irregular nuclear membranes, and fine chromatin. Multiple mitotic figures were noted. The tumoral lymphomatoid cells showed expression of CD20, Bcl2, Bcl6, CD10, and MUM1. The Ki 67 proliferation index was estimated at 80%, and they were negative for CD3 and CD5.
On the basis of histological results and the absence of other tumor lesions or localizations, the diagnosis of primary diffuse large B-cell lymphoma of orbit was retained.
The patient was referred to the hematologist-oncologist. He underwent a PET/CT scan, which showed no other localization, and he received 6 cycles of chemotherapy R-CHOP (rituximab, doxorubicin, cyclophosphamide, vincristine, and prednisolone) combined with high-dose methotrexate (administered on day 14), the total duration of treatment was three months, with a complete remission and a good tolerance to the treatment. The patient is still under regular follow-up every six months.
DISCUSSION
Orbital lymphoma can be of primary origin, or secondary in the context of a systemic disease [7]. Approximately 10–32% of orbital lymphomas represent part of disseminated lymphoma or tumor relapse [8]. Orbital lymphoma is common in elderly people, without gender predominance, the average age is 69 years [9].
On the other hand, advanced age has been shown to be a factor in poor prognosis [10]. Our patient’s advanced age is consistent with the mean age of other studies [8],[11].
Orbital lymphoma is often of B origin (97%), very few cases of primary T-cell lymphomas of the orbital lymphoma have been reported in the literature [4],[12].
Extranodal marginal zone B-cell lymphoma (EMZL) is the most common subtype (59%). It may be associated with Chlamydia psittaci infection [13]. Its prognosis is generally favorable. The 10-year survival rate can be up to 93% [14].
Diffuse large B-cell lymphoma is the second-most common NHL in orbit with the worst prognosis, high aggressiveness, and rapid visual loss (23%) [10],[15].
It consists of medium-sized to large B-cells with a diffuse growth pattern. The tumor is morphologically and molecularly heterogeneous [16] in cases of DLBCL that arise from transformation of follicular or marginal zone lymphoma; so residual follicles may be present [10]. Diffuse large B-cell lymphoma can arise, usually, de novo, or represent transformation of a less aggressive lymphoma, such as chronic lymphocytic leukemia, follicular lymphoma, marginal zone lymphoma, or nodular lymphocyte predominant Hodgkin lymphoma. It can be also associated to context of immunodeficiency caused by disease or drugs. In our case, the patient had no specific history and the DLBCL occurred de novo. Diffuse large B-cell lymphomas are CD20+. However, CD20 may be negative in biopsies taken after rituximab treatment. Alternative B-cell markers such as CD19, CD22, CD79a, and/or PAX5 should be employed to confirm B-cell differentiation and support the differential diagnosis. Ki67 is usually >50%. The tumor has few reactive T-cells [16]. T-cell markers such as CD5 are usually negative. However, some cases of DLBCL will co-express CD5 and are associated with a poorer prognosis [17]. There are two subtypes of DLBCL, termed germinal center B-cell-like (GCB) and activated B-cell-like (ABC), using Hans algorithm: CD10 positivity is an initial determining factor, and further Bcl-6 and MUM1 positivity. The end result of the Hans algorithm defines the GCB subtype as any lesion that exhibits CD10+, or CD10- Bcl6+ MUM1–,using 30% positivity as a cutoff, and ABC subtypes as any lesion CD10- Bcl6- or CD10- Bcl6+ MUM1+ [18].
Follicular lymphoma is the third most common ocular lymphoma (9%). It is a B-cell neoplasm of germinal center B-cells, composed of centrocytes and/or large transformed cells, centroblasts, usually with a pattern that is at least partially follicular [16]. It involves most frequently the orbital soft tissue, conjunctiva, and lacrimal gland. Secondary follicular lymphoma is relatively common; lymph nodes being the most common primary site. Most patients are middle-aged to older, often in their 50s or 60s, with female predominance [19].
Mantle cell lymphoma (MCL) is the fourth most common subtype of ocular adnexal lymphoma (OAL) and accounts for 5–11% of all OALs [4]. It is defined as a mature B-cell neoplasm arising from the mantle zone of lymphoid follicles, and typically composed of small to medium-sized monomorphic cells expressing CD5, SOX11, and cyclin D1. It is associated with translocations involving CCND family, most commonly CCND1. Most patients are elderly with a median age of 60–70 years, with a significant male predominance (80%).
Clinical
Ocular adnexal lymphoma is characterized by its clinical polymorphism. It can be manifested by periorbital tumor mass, exophthalmos, eye motility and visual restrictions, ocular pain, and proptosis. It can also mimic glaucoma [3]. However, Ocular and adnexal lymphoma may affect the lacrimal gland, extraocular muscles, or orbital space. It tends to mold around existing orbital structures rather than invade them. Therefore, visual loss and diplopia are rarely present [20]. In contrast, the main reason for our patient’s consultation was a gradual decrease in visual acuity associated with diplopia. Diagnosis can be easily missed on imaging and confused with other diseases due to unconventional atypical appearances including inflammation. Well-defined borders and bone changes, among others, are unconventional factors that can cause pitfalls in initial diagnosis [21]. Pathologically, immunohistochemistry is essential for the diagnosis of DLBCL and its differentiation from other large-cell orbital adnexal lymphohematopoietic tumors including granulocytic sarcoma, lymphoblastic leukemia, lymphomatoid granulomatosis, plasmablastic lymphoma, Burkitt lymphoma, anaplastic large T-cell lymphoma, natural killer/T-cell lymphoma, histiocytic sarcoma, and interdigitating dendritic cell sarcoma. Therefore, orbital lymphoma can occur at any stage of the disease and should always be considered when it comes to an elderly adult with proptosis, full eyelids, diplopia, or eye irritation, without a history of lymphohematopoietic disease [3].
Prognosis
The histopathological subtype and clinical stage of the disease are the best indicators of prognosis. Low-grade lymphomas such as marginal B-cell extranodal lymphoma and follicular lymphoma have a good prognosis, while high-grade lymphomas (large B-cell diffuse lymphoma and mantle cell lymphoma) are associated with poor prognosis. However, there are immunohistochemical and molecular markers, for DBLCL that can predict its prognosis. Particularly, expression of CD5, Epstein–Barr virus positivity and concurrent high expression of BCL2 and MYC in DLBCL (double expressors) carry an adverse prognosis. The ABC-subtype has an inferior outcome in response to standard therapies when compared to the GCB-subtype [16]. Younger age (below 60 years) and total resection are associated with increased survival. OA-uveal DLBCL demonstrated a 40.9% mortality rate by 5 years.
Ocular adnexal lymphoma can also extend to the central nervous system (CNS); however, the likelihood is relatively low, especially in unilateral disease [22].
The 5-year survival rate of DLBCL exceeds 55% [10].
Treatment
The surgery consisting of total resection, enucleation, is associated with a decrease in the risk of death; but surgery alone is not generally used because of the difficulty of visualizing all the infiltrating mass for resection with a risk of recurrence. The backbone of treatment is chemotherapy and/or radiotherapy [23],[24].
Radiation therapy can induce cataract, retinopathy, and neuropathy, as well as an intensive induction-consolidation regimen of methotrexate chemotherapy which can also induce keratopathy, maculopathy, and drug resistance. This risk led to the recommendation to reduce chemotherapy cycles with longer intervals and to add rituximab as a less toxic approach [22].
CONCLUSION
Diffuse large B-cell lymphoma is the second most common NHL in orbit, affected usually elderly patients (average age 69), with no sex predilection. It can manifest as exophthalmos, pain, decreased visual acuity, diplopia, and ptosis, and it has often a poor prognosis. Diagnosis is histopathological, based on morphology including diffuse proliferation of large atypical cells and requiring immunohistochemical study to confirm diagnosis and subtype. The treatment is multidisciplinary consisting of chemotherapy with or without radiotherapy. In front of any elderly person with exophthalmos, diplopia, or other sign of eye irritation: think about eliminating a DLBLC.
REFERENCES
1.
Verdijk RM. Lymphoproliferative tumors of the ocular adnexa. Asia Pac J Ophthalmol (Phila) 2017;6(2):132–42. [CrossRef]
[Pubmed]

2.
Eyelid, Conjunctival, and Orbital Tumors: An Atlas and Textbook. 2015. [Available at: https://www.wolterskluwer.com/en/solutions/ovid/eyelid-conjunctival-and-orbital-tumors-an-atlas-and-textbook-13758]

3.
Olsen TG, Heegaard S. Orbital lymphoma. Surv Ophthalmol 2019;64(1):45–66. [CrossRef]
[Pubmed]

4.
Holm F, Mikkelsen LH, Kamper P, et al. Ocular adnexal lymphoma in Denmark: A nationwide study of 387 cases from 1980 to 2017. Br J Ophthalmol 2021;105(7):914–920. [CrossRef]
[Pubmed]

5.
Tripathy D, Pradhan A, Mittal R. Acute orbital presentation in an activated B-cell subtype, diffuse large B-cell lymphoma: Clinicoradiological, histopathological and immunohistochemical correlation. Indian J Ophthalmol Case Rep 2021;1(3):608–10. [CrossRef]

6.
Aldaas KM, Randall C, Eftekhari K, Zhang AY. Orbital diffuse large B-cell lymphoma initially presenting as neovascular glaucoma. Ophthalmic Plast Reconstr Surg 2020;36(1):e12–3. [CrossRef]
[Pubmed]

7.
Esmaeli B, Ahmadi MA, Manning J, McLaughlin PW, Ginsberg L. Clinical presentation and treatment of secondary orbital lymphoma. Ophthalmic Plast Reconstr Surg 2002;18(4):247–53. [CrossRef]
[Pubmed]

8.
Ferry JA, Fung CY, Zukerberg L, et al. Lymphoma of the ocular adnexa: A study of 353 cases. Am J Surg Pathol 2007;31(2):170–84. [CrossRef]
[Pubmed]

9.
Stacy RC, Jakobiec FA, Herwig MC, Schoenfield L, Singh A, Grossniklaus HE. Diffuse large B-cell lymphoma of the orbit: Clinicopathologic, immunohistochemical, and prognostic features of 20 cases. Am J Ophthalmol 2012;154(1):87–98.e1. [CrossRef]
[Pubmed]

10.
Chen YQ, Yue ZF, Chen SN, Tong F, Yang WH, Wei RL. Primary diffuse large B-cell lymphoma of orbit: A population-based analysis. Front Med (Lausanne) 2022;9:990538. [CrossRef]
[Pubmed]

11.
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89(11):3909–18.
[Pubmed]

12.
Coupland SE, Hummel M, Stein H. Ocular adnexal lymphomas: Five case presentations and a review of the literature. Surv Ophthalmol 2002;47(5):470–90. [CrossRef]
[Pubmed]

13.
Ferreri AJM, Guidoboni M, Ponzoni M, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst 2004;96(8):586–94. [CrossRef]
[Pubmed]

14.
Hindsø TG, Esmaeli B, Holm F, et al. International multicentre retrospective cohort study of ocular adnexal marginal zone B-cell lymphoma. Br J Ophthalmol 2020;104(3):357–62. [CrossRef]
[Pubmed]

15.
Olsen TG, Holm F, Mikkelsen LH, et al. Orbital lymphoma – An international multicenter retrospective study. Am J Ophthalmol 2019;199:44–57. [CrossRef]
[Pubmed]

16.
Li W. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. In: Li W, editor. Leukemia. Brisbane (AU): Exon Publications; 2022. Chapter 1.
[Pubmed]

17.
Li S, Young KH, Medeiros LJ. Diffuse large B-cell lymphoma. Pathology 2018;50(1):74–87. [CrossRef]
[Pubmed]

18.
Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103(1):275–82. [CrossRef]
[Pubmed]

19.
Rasmussen PK, Coupland SE, Finger PT, et al. Ocular adnexal follicular lymphoma: A multicenter international study. JAMA Ophthalmol 2014;132(7):851–8. [CrossRef]
[Pubmed]

20.
Li EY, Yuen HK, Cheuk W. Lymphoproliferative disease of the orbit. Asia Pac J Ophthalmol (Phila) 2015;4(2):106–11. [CrossRef]
[Pubmed]

21.
Briscoe D, Safieh C, Ton Y, Shapiro H, Assia EI, Kidron D. Characteristics of orbital lymphoma: A clinicopathological study of 26 cases. Int Ophthalmol 2018;38(1):271–7. [CrossRef]
[Pubmed]

22.
Ahmed AH, Foster CS, Shields CL. Association of disease location and treatment with survival in diffuse large B-cell lymphoma of the eye and ocular adnexal region. JAMA Ophthalmol 2017;135(10):1062–8. [CrossRef]
[Pubmed]

23.
Esik O, Ikeda H, Mukai K, Kaneko A. A retrospective analysis of different modalities for treatment of primary orbital non-Hodgkin’s lymphomas. Radiother Oncol 1996;38(1):13–8. [CrossRef]
[Pubmed]

24.
Schick U, Lermen O, Unsöld R, Hassler W. Treatment of primary orbital lymphomas. Eur J Haematol 2004;72(3):186–92. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Siham Nagib - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Manal Abatourab - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Da Silva Fidélia Nihad - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ulrich Opoko - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Samira Benayad - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Faiçal Slimani - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Mehdi Karkouri - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this article.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2024 Siham Nagib et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.